- Home
- About Us
- Products & Services
- Our Services
- License Representative
- Product Registration
- Medical Device Company Establishment Service
- Renewal and License Amendment
- Miscellaneous Certificates
- Post-Marketing Surveillance
- E- Catalogue
- Medical Device Trademark Management
- GDPMD (Good Distribution Practice for Medical Device)
- Regulatory Intelligence
- research material products
- Our Services
- Articel
- Asia Regulatory Information
- Our Clients
- Gallery
- Contact US

In 2010, the Indonesian Ministry of Health issued several important regulations concerning the production of medical devices and household products. These include PERMENKES 1189/VIII/2010, which pertains to medical device production; PERMENKES 1191/VIII/2010, which addresses medical device distributors; and PERMENKES 70/2010, which focuses on household producers. With the introduction of the latest presidential regulations, No. 91/2017 and 24/2018, aimed at business acceleration and the integration of electronic licensing services, the Ministry of Health has implemented a new regulatory framework to streamline licensing services in the health sector, thereby expediting the medical equipment licensing process in Indonesia.
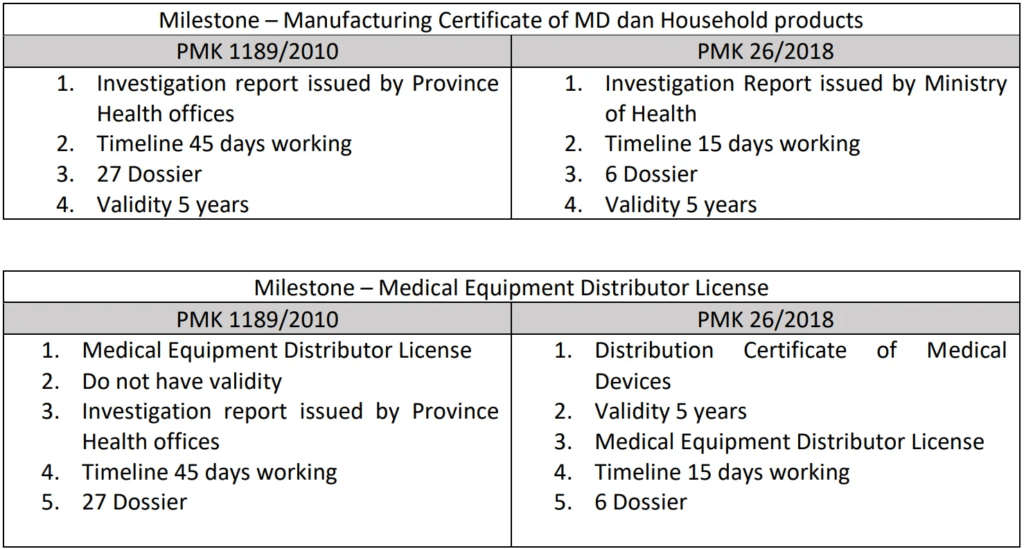
Under the previous regulations, companies involved in manufacturing and distributing medical devices were required to obtain both a manufacturing license and a medical equipment distributor license. This process involved an investigation by regional health offices based on the company’s domicile. However, the new regulation PMK 26/2018 shifts the investigative responsibility to the central government (Ministry of Health), further accelerating the licensing process. Additionally, the new regulations have reduced the requirements, shortened timelines, and established a five-year validity period for distributor licenses.
The Ministry of Health oversees approximately 33 health sectors, which include product approval for medical devices, in vitro diagnostics (IVD), household products, distribution
In line with the presidential regulations concerning integrated electronic licensing services, the Indonesian government launched a new system in July 2018 called OSS (Online Single Submission). This system mandates that all permits and licensing processes, including medical device licensing, be conducted through a single-window approach.
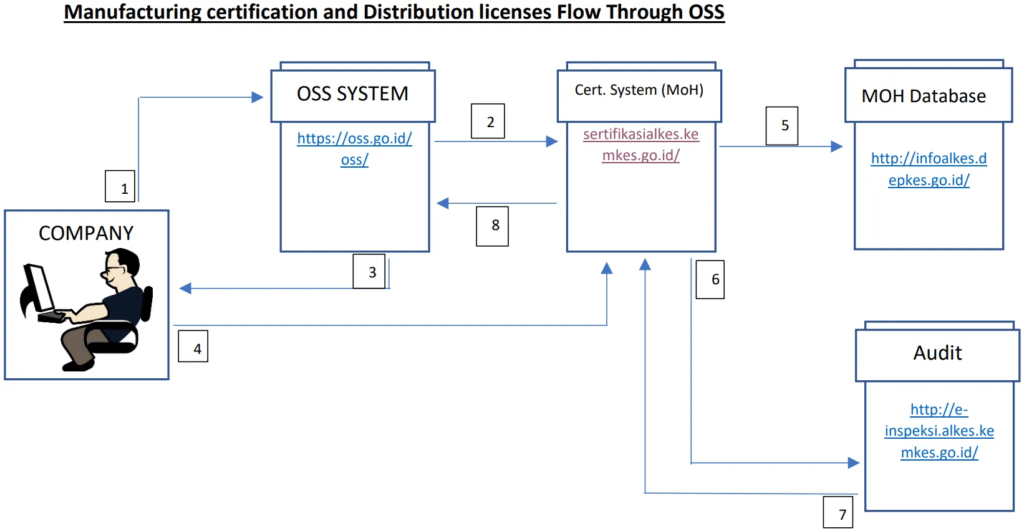
While it remains uncertain when these regulations will be fully implemented across all health sectors, changes are actively underway. According to regulation 26/2018, Chapter IV, three heads of government are authorized to issue permits: the Ministry of Health, regional governors, and regents or mayors. Specifically, the Ministry of Health is responsible for product approval licensing, manufacturing licenses, distribution licenses, and GDPMD certification.
Artikel Lainnya
-

Medical Device Business Sector Code (KBLI) Changes: Government Issues New KBLI 2025
-

Annual MoH System Maintenance and Year-End Closure Announcement
-

Obligation to Submit Internal Audit Reports for Medical Device Companies Holding GDPMD Certificates
-

Udpate List of Medical Device and PKRT Testing Laboratory Network Accredited by SNI ISO/IEC 17025:2017
-

Indonesia's Ministry of Health Recognizes Dried Blood Spot as IVD Medical Device
Our Services
- License Representative
- Product Registration
- Medical Device Company Establishment Service
- Renewal and License Amendment
- Miscellaneous Certificates
- Post-Marketing Surveillance
- E- Catalogue
- Medical Device Trademark Management
- GDPMD (Good Distribution Practice for Medical Device)
- Regulatory Intelligence
- IDAK (Izin Distributor Alat Kesehatan)