- Home
- About Us
- Products & Services
- Our Services
- License Representative
- Product Registration
- Medical Device Company Establishment Service
- Renewal and License Amendment
- Miscellaneous Certificates
- Post-Marketing Surveillance
- E- Catalogue
- Medical Device Trademark Management
- GDPMD (Good Distribution Practice for Medical Device)
- Regulatory Intelligence
- research material products
- Our Services
- Articel
- Asia Regulatory Information
- Our Clients
- Gallery
- Contact US
Product Registration
The classification of medical devices in Indonesia is divided into four categories, namely A, B, C, and D following the AMDD (Asean Medical Device Regulation) regulation.
Our Services
- License Representative
- Product Registration
- Medical Device Company Establishment Service
- Renewal and License Amendment
- Miscellaneous Certificates
- Post-Marketing Surveillance
- E- Catalogue
- Medical Device Trademark Management
- GDPMD (Good Distribution Practice for Medical Device)
- Regulatory Intelligence
- IDAK (Izin Distributor Alat Kesehatan)
Product Registration
The product registrations that we offer are as follows:
Classification of medical devices in Indonesia is considered into four categories, will be A, B, C, and D following AMDD (Asean Medical Device Regulation) regulation. Class A is a low-risk medical device, Class B is a low-to-moderate-risk medical device, Class C is a moderate-to-high-risk medical device, and Class D is a high-risk.
In accordance with the guidelines for medical device license approval issued by the Ministry of Health of the Republic of Indonesia, Product application requirements are grouped into 5 forms that must be completed by the applicant including, Form A (administrative requirements), Form B (product information requirements), Form C (specification information). and quality assurance), Form D (instructions for use), Form E (post market evaluation).
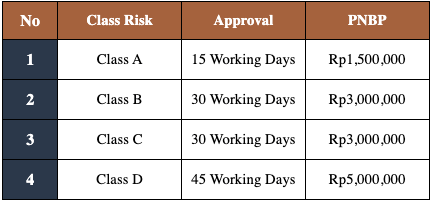
The government fees or PNBP (Non-Tax State Revenue) and the time line approval based on PMK 62/2017 are as follows:
- Medical Device Registration
- IVD Medical Device Registration
- Household health Supplies Registration
Classification of medical devices in Indonesia is considered into four categories, will be A, B, C, and D following AMDD (Asean Medical Device Regulation) regulation. Class A is a low-risk medical device, Class B is a low-to-moderate-risk medical device, Class C is a moderate-to-high-risk medical device, and Class D is a high-risk.
In accordance with the guidelines for medical device license approval issued by the Ministry of Health of the Republic of Indonesia, Product application requirements are grouped into 5 forms that must be completed by the applicant including, Form A (administrative requirements), Form B (product information requirements), Form C (specification information). and quality assurance), Form D (instructions for use), Form E (post market evaluation).
- Form A (administrative requirements)
- Form B (product information requirements)
- Form C (specification and quality assurance information)
- Form D (instructions for use)
- Form E (post market evaluation)
The government fees or PNBP (Non-Tax State Revenue) and the time line approval based on PMK 62/2017 are as follows: